Medical device design, manufacturing, regulatory, and innovation.
Use design controls for innovation
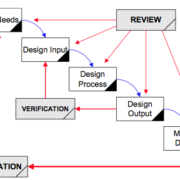
/in Design and Manufacturing, Medical Devices /by jasonpartinThis article helps teams “design a pen” using design controls in a way that sparks innovation. I wrote it for the biotech or medical device industries but the concepts apply to any company that must share work among a team. We go from defining user needs to transferring design into manufacturing.

Medical Device Design Controls
/in Design and Manufacturing, Medical Devices /by jasonpartin
5 minute read.
The European MDR: learn from my mistakes
/in EU-MDR, Medical Devices /by jasonpartinThis article is to help you learn the MDR using a product I co-invented and commercialized in 2004, demonstrating how to apply modern MDR regulations. I describe that product in another article. This article helps you learn from my mistakes.
Design Control: learn from my mistakes
/in Design and Manufacturing, Medical Devices /by jasonpartin
Risk Management: learn from my mistakes
/in Medical Devices, Risk Management /by jasonpartinI describe that product in another article. This article helps you learn from my mistakes.

Medical Devices: learn from my mistakes
/in Medical Devices /by jasonpartinThat sounds like a success, but years later I learned that our product caused pain and suffering for some patients and added useless healthcare costs to everyone.
In this article you can choose your adventure, choosing which you’d like to see addressed after 15 more years of experience in:
- Design Controls
- Risk Management
- Government Regulations: FDA and EU-MDR
- Entrepreneurship

Risk Control & Risk-Benefit
/in EU-MDR, Medical Devices, Risk Management /by jasonpartinThis article explains Risk / Benefit analysis and Risk Control methods using performances of Harry “The Hat” Anderson, a comedian, actor, and magician famous for visual humor.

Make state of the art medical devices
/in Design and Manufacturing, EU-MDR, Medical Devices, Risk Management /by jasonpartinThe new European Union Medical Device Regulation will protect patient safety by requiring healthcare companies to make products that are “state of the art,” a term that’s easily misunderstood. This law is best explained with an example from cars, comparing today’s state of the art with to the 1980’s television series Knight Rider, where David Hasselhoff fought crime in a talking, self-driving car.
Take this quiz to see if you’re ready for MDSAP
/in Medical Devices /by jasonpartinBy January 2019, the Canadian government will require all companies selling medical devices in Canada to be certified under the Medical Device Single Audit Program (MDSAP). If you’re familiar with MDSAP, this article quizzes your knowledge of key concepts. If it’s new to you, read how to prepare for MDSAP.