What would it look like?



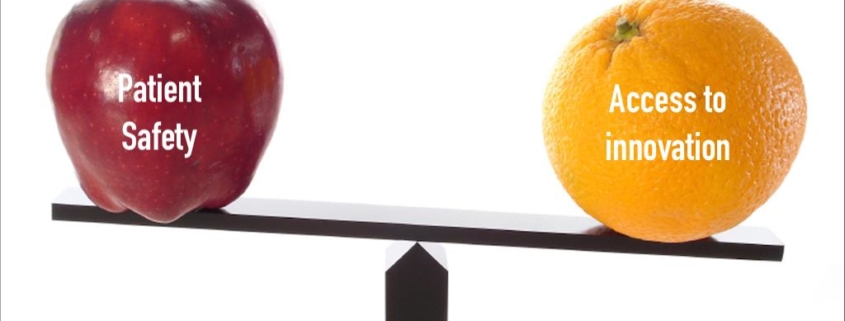
The United States Food and Drug Administration hasn’t updated its regulations since 1997; they recently announced they are beginning that process and are soliciting feedback from companies and the public.The FDA reviews new technologies and inspects existing manufacturing facilities, hopefully to ensure consistency in products.FDA or EUMDR approval does not automatically mean the devices are cost-effective or proven on a large scale, consider all complex factors of manufacturing and use, or are beneficial compared to alternative approaches or competitive products.FDA approval is usually based on “predicate devices,” which means that if you’re similar to something already approved you can get also get approved; you can choose which predicate device you use for comparison and you don’t have to make it safer.